Previous research has indicated that resistance of colorectal cancer to chemotherapy can be a consequence of a protein called BCL-XL. This critical protein protects colorectal cancer stem cells from therapy-induced death. In the lab, the activity of BCL-XL in cancer stem cells can be counteracted by specific targeted molecules (BH3 mimetics) that block BCL-XL and restore therapy sensitivity and cell killing. However, blocking BCL-XL by BH-3 mimetics cannot be used as a therapy for patients because the lifespan of blood platelets also depends on BCL-XL, causing unwanted toxicity. In search of better treatment strategies, an international research team led by Prof. J.P. Medema performed a drug screen to identify compounds that would greatly support the blocking activity of BH-3 mimetics while avoiding toxicity for blood platelets.
Screening for synergy
The scientists performed a large drug screen involving many different compounds that had already been approved by the US Federal Drug Administration for therapeutic applications in patients. Prashanthi Ramesh, a PhD student at Cancer Center Amsterdam and first author on the scientific paper explains: “We exposed CRC cultures to low levels of BH-3 mimetics that barely impaired the growth of these cells in the lab. In addition, we applied a drug library and found molecules that effectively killed the cancer cells in combination with non-toxic BCL-XL inhibition.”
The effective cell killing after combining low-dose BH-3 mimetic and identified drugs is a consequence of a synergistic effect, which provides a much stronger combined anti-cancer activity than could have been predicted by adding up the effects of each separate drug.
Escaping cell death
Looking into the mechanism of how this combination of drugs effectively killed the CRC cells, the researchers found that the cancer cells hijack a pathway that normally seems to protect the viability of the intestinal lining. Prashanthi: “Cells that are closer to dying quickly secrete a rescue signal that makes them stronger and more resistant to cell death, and also provides protection for neighboring cells. Blocking this rescue signal makes cancer cells more vulnerable to BCL-XL-inhibition and cell death”.
This new treatment strategy showed promise in the lab by effectively killing colon cancer cells while sparing normal healthy cells in 3D human organoid cultures and mice with CRC. Still, more extensive pre-clinical testing is necessary before this treatment strategy can be offered to patients.
Prashanthi concludes: “The escape mechanism we discovered is also relevant in other solid cancers. It is important to carefully consider the consequences of inducing cell death through therapy, as cancer cells primed for death can secrete signals that ultimately confer treatment resistance.”
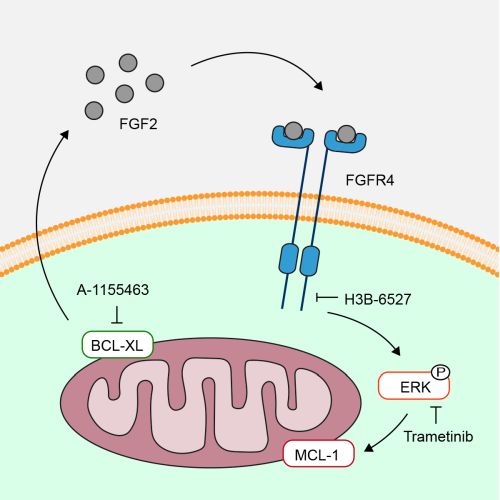 Figure. Inhibition of the anti-cell death factor BCL-XL by small molecules triggers an innate cell response to quickly secrete FGF2 to counteract pending cell death.
Figure. Inhibition of the anti-cell death factor BCL-XL by small molecules triggers an innate cell response to quickly secrete FGF2 to counteract pending cell death.
For more information contact Prashanthi Ramesh, or read the scientific publication: Ramesh, P., et al. (2022) BCL-XL inhibition induces an FGFR4-mediated rescue response in colorectal cancer. Cell Reports 38, 110374. https://doi.org/10.1016/j.celrep.2022.110374
Involved researchers at Cancer Center Amsterdam – Amsterdam UMC:
Prashanthi Ramesh
Lidia Atencia Taboada
Le Zhang
Jan Paul Medema
This research was supported by Oncode Institute, the Dutch Cancer Society (KWF), and Le Zhang was supported by a scholarship of the Chinese Scholarship Council.
Text by Henri van de Vrugt