Drugs that target the epidermal growth factor receptor (EGFR) have become standard treatment in metastatic colorectal cancer. However, not every patient responds to current EGFR inhibitors. To understand why, a research team lead by Connie Jimenez employed innovative mass spectrometry-based techniques to investigate the molecular basis of resistance and sensitivity, and identify alternative drug targets to overcome resistance. The work was published in Science Translational Medicine.
Epidermal growth factor receptor (EGFR) is a kinase protein receptor controlling numerous signaling pathways critical for cell growth, migration, and proliferation. EGFR is often over-expressed in tumor cells, a factor that enhances tumor growth, invasion, and metastasis. Consequently, EGFR-targeted therapies have become standard treatment for several types of cancer, including metastatic colorectal cancer (mCRC).
However, not every patient responds to current EGFR inhibitors. To understand why, a research team lead by Prof. Connie Jimenez, together with colleagues from Candiolo Cancer Institute, Turin, employed innovative mass spectrometry-based techniques to compare the proteomic and phosphoproteomic landscape of samples from sensitive and resistant (cetuximab) tumors.
The team examined 30 genomically and pharmacologically characterized tumors from patients using patient-derived xenograft models, in which tumor tissues from patients are implanted into laboratory mice.
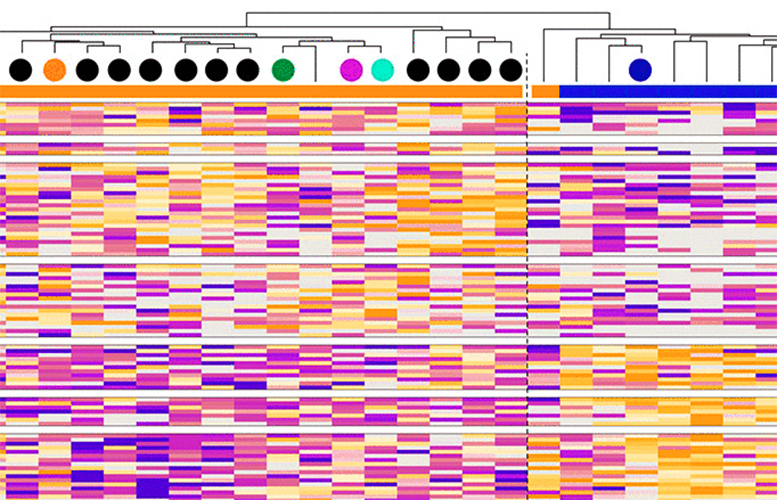
This analysis unveiled distinctive response signatures in the proteome as well as the phosphoproteome. The proteome encompasses the whole set of proteins within a cell at a given time, while phosphoproteome analysis examines protein phosphorylation, a key modification (facilitated by kinases) that regulates protein function in most cellular processes. Importantly, phosphorylation also regulates the activity of kinase enzymes themselves, forming a regulatory loop that is critical in maintaining the proper functioning of a cell.
"By delving into the intricate interaction networks of proteins and phospho-sites, we’ve uncovered a distinct biology associated with cetuximab sensitivity and resistance,” says Robin Beekhof, first author and PhD candidate at Cancer Center Amsterdam. “For example, we found that sensitive tumors displayed abundant phospho-tyrosine phosphorylation in certain proteins - like cell junction proteins - while resistant ones showed heightened MAPK and AKT signaling.”
Furthermore, the phosphoproteomics data pointed towards known kinases that drive cancer and other potential targets in resistant tumors.
“Our analysis also revealed hyperactive SRC and Ephrin family kinases in cases with genomically unexplained resistance. Inhibition of these kinases, alone or in combination with cetuximab, resulted in growth inhibition ex vivo, in tumor-derived organoids, and in vivo,” explains Robin.
These results underscore the vast possibilities of phosphoproteomics in cancer research and treatment.
“Our findings should motivate the development of phosphoproteomics-based companion diagnostics. The better we understand the proteome and phosphoproteome landscape, the more we can tailor and personalize anticancer medicine for each individual patient,” says Prof. Connie Jimenez.
For more information on (phospho)proteomics at AmsterdamUMC, visit www.oncoproteomics.nl, or contact Prof. Connie Jimenez.
The scientific publication is available here:
Robin Beekhof, et al. (2023) Phosphoproteomics of patient-derived xenografts identifies targets and markers associated with sensitivity and resistance to EGFR blockade in colorectal cancer. Sci. Transl. Med. 15,eabm3687. https://doi.org/10.1126/scitranslmed.abm3687
People involved at Amsterdam UMC:
Robin Beekhof
Franziska Böttger
Alex Henneman
Jaco Knol
Thang Pham
Richard de Goeij-de Haas
Sander Piersma
Mariette Labots
Connie Jimenez
Funders involved
This study was supported by the Dutch Cancer Society grant KWF 12516 awarded to Prof. C. Jimenez. The team also thanks Cancer Center Amsterdam and René Vogels Stichting for providing a travel grant for R. Beekhof. Additionally, they acknowledge the contributions of VitrOmics Healthcare Services (VHS), Cancer Center Amsterdam, and the Netherlands Organisation for Scientific Research (NWO- Middelgroot project number 91116017) to the mass spectrometry infrastructure, as well as Surfsara for their computing infrastructure (reference e-infra180166).
Text by Laura Roy.
This article was created for Cancer Center Amsterdam.
© 2023 New Haven Biosciences Consulting– All rights reserved.