SYT1-associated disorder is a neurodevelopmental disorder characterized by neurodevelopmental delay and autistic features. In a new study published in Molecular Psychiatry Maaike van Boven and Niels Cornelisse from the Functional Genomics department at the Center for Neurogenomics and Cognitive Research and Human Genetics at Amsterdam UMC, in collaboration with Petra Zwijnenburg (Human Genetics, Amsterdam UMC), show that neurotransmitter release is less synchronized in neurons expressing a novel disease mutation associated with the disorder. This sheds new light on the molecular causes of neurodevelopmental disorders and could lead to new therapeutic targets in the future.
Timing is key
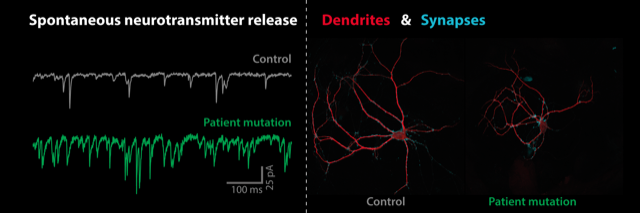
Precise timing of neurotransmitter release is essential for information processing in the brain. Children with Syt1-associated disorder carry genetic mutations in Synaptotagmin-1 (SYT1), a gene involved in the regulation of neurotransmitter release in the synaptic connections between nerve cells. Van Boven and colleagues show that a novel mutation in a patient with SYT1-associated disorder impairs the ability of Syt1 to control the precise timing of neurotransmitter release from synapses.
Neurotransmission is less synchronized during electrical stimulation, while spontaneous release of neurotransmitter in-between stimulations is drastically increased. Neurons react to this by shortening their dendrites, resulting in fewer synaptic connections. Interestingly, this cellular phenotype is different from the reduced synaptic strength found for patient mutations at other locations in SYT1. This shows that different patient mutations in the same gene can lead to diverse cellular defects, and suggests personalized therapeutic strategies are required for individual patients.
Further research
The Cornelisse lab will use stem cell technology to further investigate the link between cellular disease mechanisms and brain activity measured with EEG in patients, in collaboration with the Brainmodel consortium and the N=You Neurodevelopmental Precision Center at Amsterdam UMC. On the long term, this could lead to improved cellular diagnostics and the best possible tailor-made treatment for individual patients.

