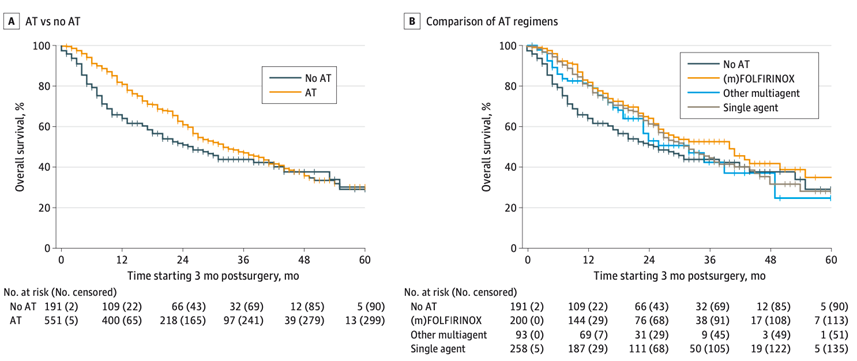
This international observational study including 767 patients – from 48 centers from Europe, United States, and Japan – who underwent resection of localized pancreatic adenocarcinoma following preoperative (m)FOLFIRINOX chemotherapy demonstrated that adjuvant chemotherapy was independently associated with prolonged overall survival. The effect of adjuvant chemotherapy on overall survival seemed to attenuate after approximately 30 months.
The interaction analysis to assess treatment effects across sub-groups was not significant, suggesting that all patients may benefit from adjuvant chemotherapy. However, the forest plot and interaction test suggest that the effect of adjuvant chemotherapy is less in patients treated with >8 cycles preoperative (m)FOLFIRINOX, patients with having radiological response after preoperative (m)FOLFIRINOX, and ypN0. Randomized controlled trials are needed to give a definite answer on the value of adjuvant chemotherapy, particularly in the above-mentioned sub-groups. Furthermore, the optimal number of cycles perioperative chemotherapy should be addressed in future randomized trials.
Compared to no adjuvant chemotherapy, both adjuvant (m)FOLFIRINOX and other multi-agent adjuvant regimens were associated with prolonged overall survival, whereas single-agent adjuvant chemotherapy was not. This finding is highly relevant for patient counseling and shared decision-making, especially in patients with an impaired performance status or those who experienced significant toxicity by preoperative (m)FOLFIRINOX.
Researchers involved:
*marked in bold when part of Amsterdam UMC
Thomas F. Stoop, MD1-3*; Toshitaka Sugawara, MD, PhD1,4*; Atsushi Oba, MD, PhD1,4,5*; Isabel M. Feld, BSc2,3; Stijn van Roessel, MD, PhD2,3; Eran van Veldhuisen, MD, PhD2,3; Y.H. Andrew Wu, MD1,6,7; Jo Nishino, MSc, PhD8; Mahsoem Ali, BSc2,3; Adnan Alseidi, MD, EdM9; Alain Sauvanet, MD, PhD10; Antonello Mirabella, MD, PhD11; Antonio Sa Cunha, MD, PhD12; Arto Kokkola, MD, PhD13; Bas Groot Koerkamp, MD, PhD14; Daniel Pietrasz, MD, PhD12; Dyre
Kleive, MD, PhD15; Giovanni Butturini, MD, PhD16; Giuseppe Malleo, MD, PhD17; Hanneke W.M. van Laarhoven, MD, PhD, PhD3,18; Isabella Frigerio, MD, PhD16; Jeanne Dembinski, MD10; Jin He, MD, PhD6,7; Johan Gagnière, MD, PhD19; Jörg Kleeff, MD, FACS20; Jose M. Ramia, MD, PhD21; Keith J. Roberts, MD, PhD, FRCS22; Knut J. Labori, MD, PhD15; Marco V. Marino, MD11; Massimo Falconi, MD23; Michael B. Mortensen, MD, PhD24; Mickaël Lesurtel, MD, PhD12; Morgan Bonds, MD9; Nikolaos Chatzizacharias, MD, PhD22; Oliver Strobel, MD, PhD25,26; Olivier Turrini, MD, PhD27; Oonagh Griffin, RD, PhD28; Oskar Franklin, MD, PhD1,29; Per Pfeiffer, MD, PhD30; Richard D. Schulick, MD, MBA1; Roberto Salvia, MD, PhD17; Roeland F. de Wilde, MD, PhD14; Safi Dokmak, MD, PhD10; Salvador Rodriguez Franco, MD1; Simone Augustinus, MD, PhD2,3; Stefan K. Burgdorf, MD, PhD31; Stefano Crippa, MD, PhD23; Thilo Hackert, MD, PhD25,32; Timo Tarvainen, MD13; William R. Burns, MD, PhD6,7; Wells Messersmith, MD33; Johanna W. Wilmink, MD, PhD3,18; Richard A. Burkhart, MD6,7#; Marco Del Chiaro, MD, PhD, FACS1#; Marc G. Besselink, MD, MSc, PhD2,3# for the Scientific Committee of the European-African Hepato-Pancreato-Biliary Association (E-AHPBA) & International Collaboration on Advanced Pancreatic Cancer.

