Hyperactivation of the Wnt signaling pathway is a hallmark of colorectal cancer (CRC), driving cancer stem cell renewal and tumor growth. However, targeting the Wnt pathway for therapeutic purposes has been challenging due to its essential role in normal stem cell regeneration and tissue integrity maintenance. Increasing evidence suggests that long non-coding RNAs (lncRNAs) are powerful regulators of biological processes and are frequently deregulated in cancer. Moreover, their tissue- and tumor-specific expression makes them promising candidates for the development of novel cancer therapies.
Through CRISPR inhibition screens, researchers identified critical lncRNAs involved in CRC stem cell function and tumor progression, with LINC02418 standing out due to its CRC-specific expression. Analysis of human embryonic tissues showed that LINC02418 is active during early gut development. These findings suggest that CRC cells exploit a developmental program to reactivate LINC02418 during cancer progression. Mechanistically, LINC02418 plays a crucial role in maintaining high MYC expression levels by acting through a competing endogenous RNA network (ceRNET), which sequesters miR-24 and thereby prevents MYC repression.
To translate the findings, a collaboration with Alnylam Pharmaceuticals was established to develop siRNA-based therapies targeting LINC02418. In vivo testing of lipid nanoparticles-formulated siRNAs demonstrated that silencing LINC02418 markedly reduced tumor growth in xenograft models.
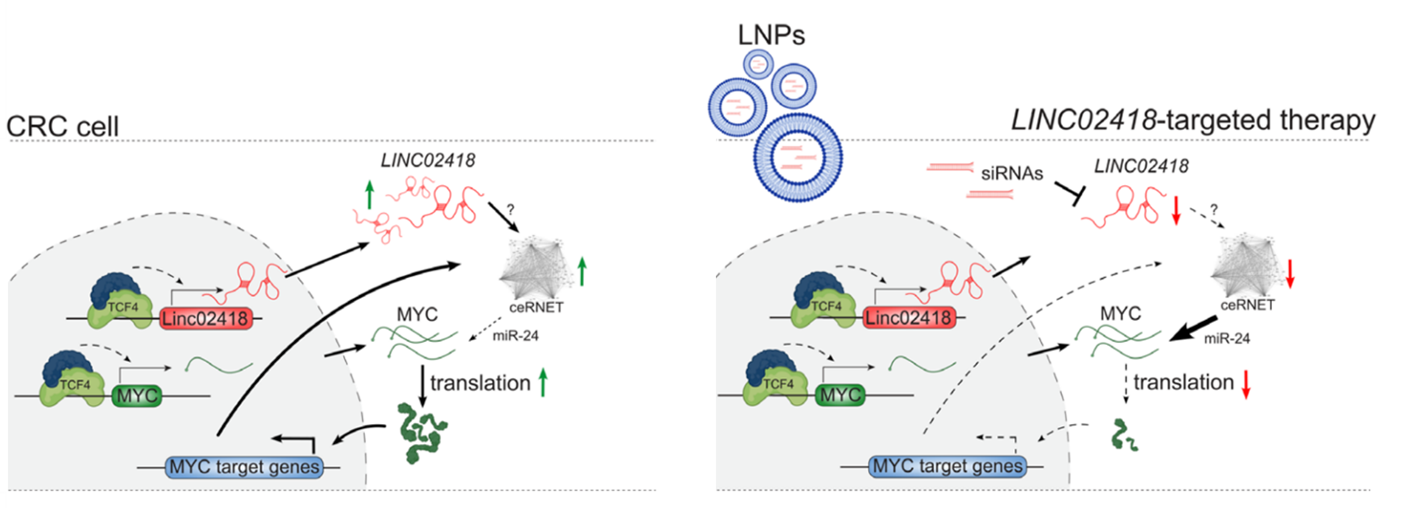
The study paves the way for the development of a new class of anticancer agents that target the long non-coding RNA landscape downstream of various oncogenic pathways. The therapeutic potential of lncRNAs is enhanced by their tissue and context specificity, offering an advantage over protein-coding mRNAs and potentially reducing toxicity in non-target tissues. Recent advancements in RNA interference as a therapeutic approach for a growing number of diseases further support the promise of this strategy, as demonstrated by our proof of concept for siRNA-based delivery to tumors.
For further information, contact: n.leveille@amsterdamumc.nl
This research was supported by the Dutch Cancer Society (KWF#12228, #15052). For more details:
Identifying colorectal cancer-specific vulnerabilities in the Wnt-driven long non-coding transcriptome | Gut
Research institutes
Cancer Center Amsterdam
Tytgat Institute for Liver and Intestinal Research
German Cancer Research Center (DKFZ)
Public-Private Partnership
Alnylam Pharmaceuticals