Protein homeostasis or proteostasis is essential for cellular function and viability but is continuously challenged by internal and environmental stressors. Therefore, cells evolved highly efficient quality control systems to adapt to these challenges. Transient reduction of protein synthesis, mediated by the endoplasmic reticulum (ER) kinase PERK, is an essential survival response during proteostatic stress. Accordingly, PERK deficiency is accompanied by severe defects in peripheral tissues in mice and men.
The paradox of neurons and PERK impairment
Neurons are long-lived post-mitotic cells that continue to develop extensive specialized properties during their lifetimes. Hence, proteostasis is particularly important for these cells. Paradoxically, neurons appear largely unaffected by PERK impairment.
Backup strategy: control translation
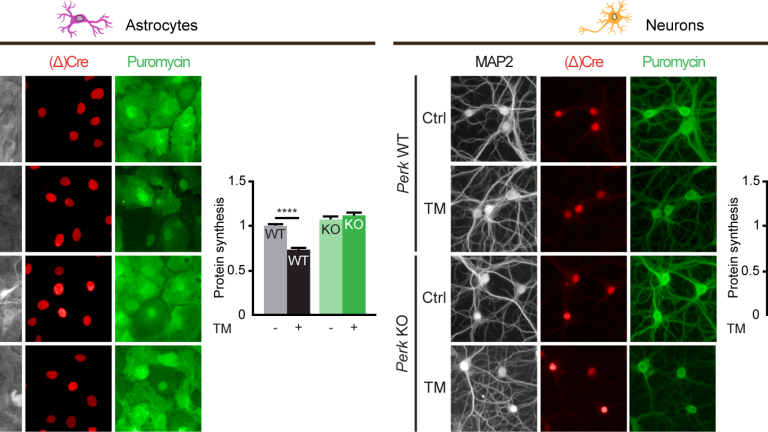
In the present study, Wolzak et al. studied PERK-deficient neurons to find the explanation for the longstanding unresolved neuronal resilience to PERK deficiency. Unexpectedly, they demonstrate that - unlike other cell types including astrocytes - PERK-deficient neurons fully retain the capacity to reduce protein synthesis during ER stress. Using a large variety of interventions and analysis methods they identify the neuron-specific backup strategy to control translation. This backup strategy is driven by two unrelated molecular pathways, one mediated by the kinase HRI and the other by the RNase angiogenin (ANG).
Understanding the triggers of neurodegeneration
Aberrant activation of PERK has been implicated in neurodegenerative diseases (reviewed in Scheper & Hoozemans, 2015) and it has been shown that PERK inhibition strongly ameliorates neurodegeneration in mouse models. Therefore, the present study provides crucial information for a better understanding of how proteostatic disturbance triggers neurodegeneration and can be targeted for disease intervention.
For the full article, published open access, see The EMBO Journal.