Two recent nationwide studies conducted in the Netherlands provide crucial insights into the real-world effectiveness of two new guideline-recommended HIV treatment options: long-acting injectable (LAI) cabotegravir plus rilpivirine, and the daily oral medication doravirine as part of a standard-of-care 3-drug regimen. While prior research has largely relied on clinical trials, these studies represent the first comprehensive real-world analyses of these therapies, offering valuable data on their effectiveness and tolerability.
Overview of the Studies
The studies, both published in The Lancet HIV, examined the effectiveness of these new treatment options in routine HIV care:
- Study 1 (January 2025) investigated the bi-monthly LAI cabotegravir and rilpivirine, available in the Netherlands since 2021.
- Study 2 (September 2024) assessed daily oral treatment with doravirine, which has been available in the Netherlands since 2018.
Both studies are based on data collected by Stichting hiv monitoring. Stichting hiv monitoring prospectively collects data of 96% of people with HIV in care in the Netherlands.
Study 1: Long-Acting Injectables (LAI) – Cabotegravir and Rilpivirine
Led by Dr. Vita Jongen (Stichting hiv monitoring SHM, Public Health Services Amsterdam, and member of the Amsterdam institute for Immunology and Infectious diseases), the first study examined whether switching to LAI cabotegravir and rilpivirine provides a viable alternative to daily oral HIV treatments. Many people with HIV face adherence challenges due to stigma, daily pill burden, or forgetfulness, making an injectable option particularly appealing.
Switching to LAI cabotegravir and rilpivirine did not result in a higher risk of virological failure among individuals without prior HIV treatment failure. The real-world effectiveness aligns with clinical trial outcomes, confirming its potential as a switch option for individuals maintaining virological suppression with standard oral antiretroviral therapy (ART).
LAI treatments offer a promising alternative for individuals struggling with daily oral regimens, addressing issues related to stigma and pill fatigue. The findings reinforce the potential of LAI therapy in routine HIV care, expanding patient-centered treatment options.
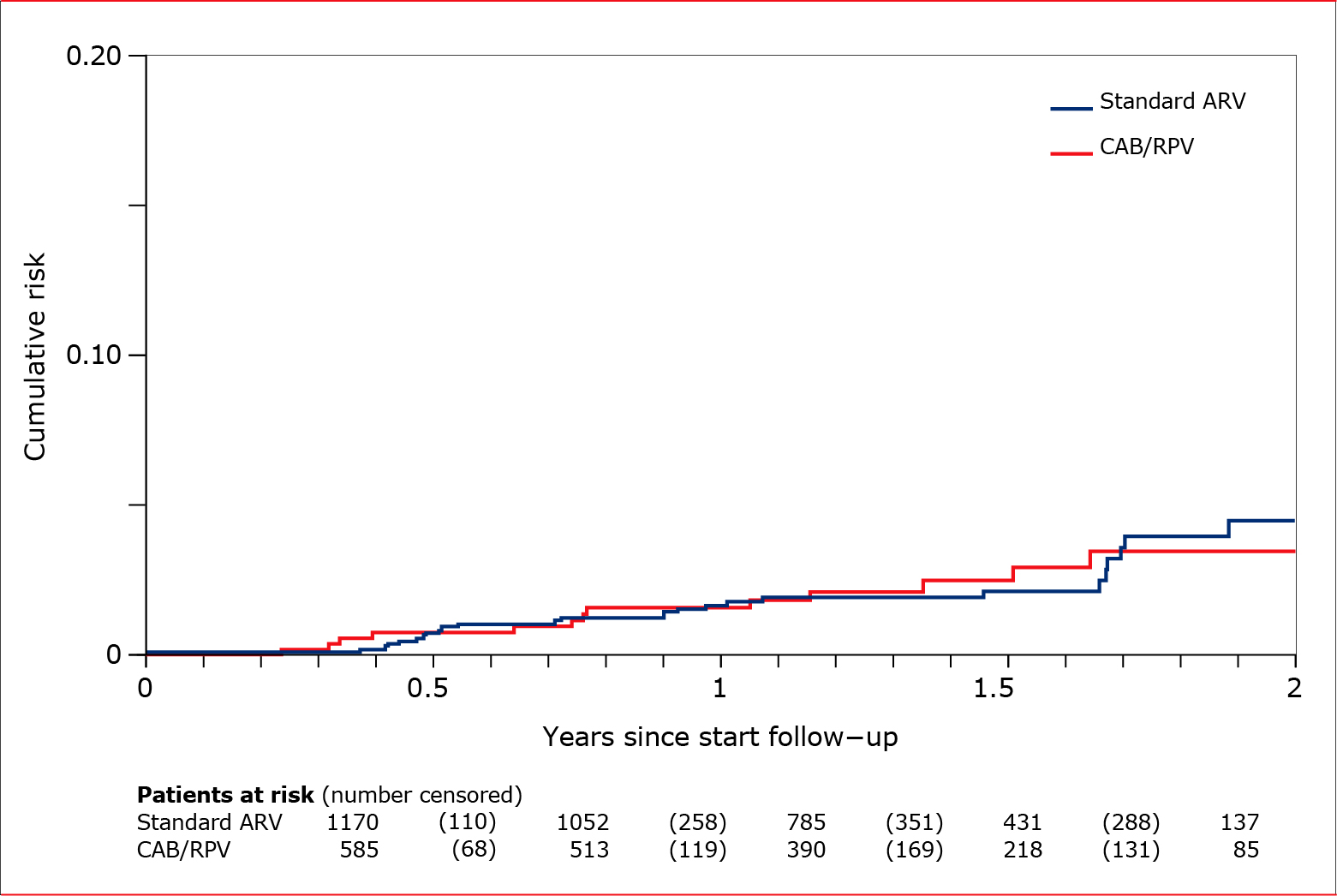 Fig 1. Study 1. Estimated cumulative risk of loss of virological control among patients using LAI cabotegravir and rilpivirine and patients using a standard, oral, antiretroviral therapy. The table shows the number of patients still at risk of losing virological control at each time point—those who had not been censored or experienced loss of control earlier. Numbers in brackets indicate patients who lost virological control between two time points. Four cases occurring after two years of follow-up are not shown in the graph.
Fig 1. Study 1. Estimated cumulative risk of loss of virological control among patients using LAI cabotegravir and rilpivirine and patients using a standard, oral, antiretroviral therapy. The table shows the number of patients still at risk of losing virological control at each time point—those who had not been censored or experienced loss of control earlier. Numbers in brackets indicate patients who lost virological control between two time points. Four cases occurring after two years of follow-up are not shown in the graph.
Study 2: Doravirine-Based Oral Treatment
The second study, led by Dr. Patrick Oomen (University Medical Center Utrecht) and Dr. Ferdinand Wit (Amsterdam UMC, Stichting HIV Monitoring, and member of the Amsterdam institute for Immunology and Infectious diseases), explored the effectiveness of doravirine-based daily oral ART. Doravirine is recognized for its favorable dosing schedule, flexibility of intake, absence of food restrictions, low drug-drug interaction potential, potent antiviral activity against HIV strains harboring common NNRTI-resistance associated mutations.
In a real-world settings, switching to doravirine-based treatment among virologically well-suppressed individuals with no history of virological failure was non-inferior in effectiveness and tolerability compared to continuing non-doravirine-containing regimens.
Doravirine provides a safe and effective switch option for individuals experiencing side effects, tolerability issues, or drug-drug interactions with other oral ART regimens. Its use can enhance treatment adherence and quality of life for people with HIV seeking a more convenient and flexible daily regimen.
Conclusion
These nationwide studies confirm the effectiveness of both long-acting injectable and doravirine-based oral HIV treatments in real-world settings. The findings support the expansion of treatment options for people with HIV, emphasizing the importance of personalized approaches to HIV management. LAI therapy offers a crucial alternative for those facing adherence challenges with daily oral pills, while doravirine provides a reliable option for individuals seeking improved tolerability and flexibility.
This article is part of a two-part series in collaboration with the Stichting hiv Monitoring (SHM). It focuses on the practical application of two innovations in HIV treatment in the Netherlands.
In the other article, we explore the current state of HIV treatment in the Netherlands, key considerations for physicians, and future developments in HIV care together with Prof. Marc van der Valk, HIV treatment specialist at the Amsterdam UMC.
For more information contact Vita Jongen (v.w.jongen@amsterdamumc.nl) or Ferdinand Wit (f.w.wit@amsterdamumc.nl) or read the scientific publications below:
- Effectiveness of bi-monthly long-acting injectable cabotegravir and rilpivirine as maintenance treatment for HIV-1 in the Netherlands: results from the Dutch ATHENA national observational cohort.
- Real-world effectiveness and tolerability of switching to doravirine-based antiretroviral therapy in people with HIV: a nationwide, matched, prospective cohort study.
Text: Esmée Vesseur (AI&I) and Sacha Boucherie (SHM)
Amsterdam UMC researchers involved:
Study 1: Effectiveness of bi-monthly long-acting injectable cabotegravir and rilpivirine as maintenance treatment for HIV-1 in the Netherlands
Vita Jongen1,2,3, PhD, Senior researcher
Ferdinand Wit1,2, PhD, Senior researcher
Anders Boyd1,2, PhD, Biostatistician and Postdoc resesarcher
Kim Sigaloff1, PhD, Principle Investigator
Marc van der Valk1,2, Professor of Internal Medicine
Study 2: Real-world effectiveness and tolerability of switching to doravirine-based antiretroviral therapy in people with HIV: a nationwide, matched, prospective cohort study
Ferdinand Wit1,2, PhD, Senior researcher
Marc van der Valk1,2, Professor of Internal Medicine
1Amsterdam Institute for Immunology and Infectious diseases (AI&I), Amsterdam UMC, the Netherlands
2Stichting hiv monitoring, Amsterdam, the Netherlands
3Department of Infectious Diseases, Public Health Service Amsterdam (GGD), Amsterdam, The Netherlands
Funding
The ATHENA Cohort is managed by the Stichting hiv monitoring and is funded by the Dutch Ministry of Health, Welfare and Sport (VWS) through a grant from the Centre for Infectious Disease Control at the National Institute for Public Health and the Environment (RIVM).
Learn more about the collaborative HIV research of Stichting hiv monitoring and the Amsterdam institute for Immunology and Infectious diseases:
- Progress and Challenges in HIV Care for Transgender Women in the Netherlands: Insights from an 11 year Study (October 2024)
- Despite access, one-third of people with HIV and hepatitis C (HCV) not treated for HCV (April 2023)
- Improved survival by screening for anal cancer precursors in people with HIV (January 2023)