The results of their study were published in Gut last week.
Novel directions in the search and development of drugs for inflammatory bowel disease (IBD) are required, as the therapeutic ceiling may have been reached in trials which are primarily based on novel insights into the inflammatory cascade. Off-label prescription includes the use of drugs outside the licensed indication, route of administration, dosage or patient population. In IBD, about one-third of the patient population is exposed to off-label drugs, and one-fifth of all IBD prescriptions are marked as off-label.
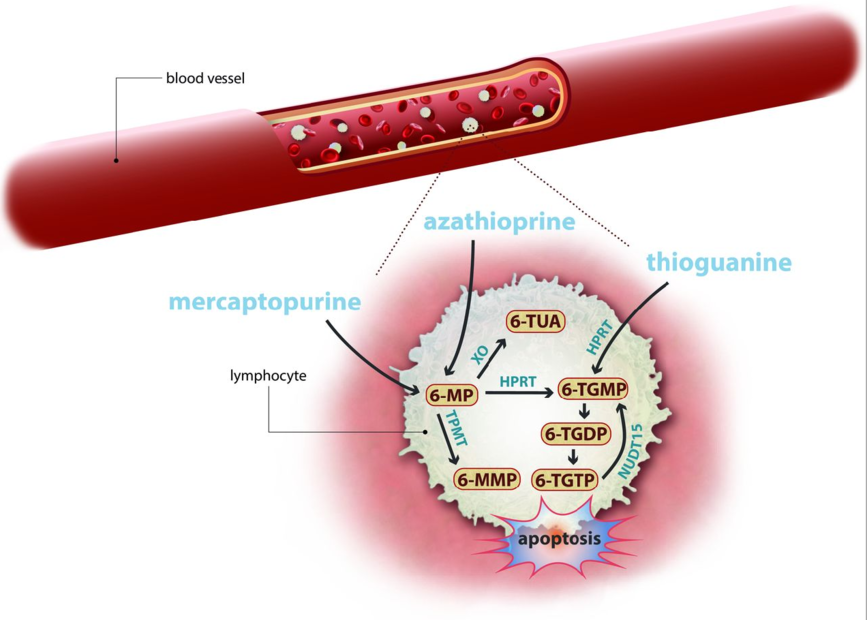
Drug rediscovery (or repurposing and repositioning) comprises the concept that a (registered) drug may be rationally and controllably used in another disease or condition than originally intended. The rationale for the use of the prodrug thioguanine (TG) in IBD is based on relatively straightforward drug metabolism, leading to one-step generation of pharmacologically active phosphorylated nucleotides and of less (potentially toxic) other metabolites. This is accompanied by better drug tolerance with (at least) similar effectiveness, compared with the more conventional thiopurine prodrugs azathioprine and mercaptopurine.
After 20 years, rediscovery of TG led to formal registration for use in patients with IBD in the Netherlands. Hurdles that hamper drug rediscovery initiatives include the lack of a protocol/blueprint for registration, limited market exclusivity after approval, and the absence of any relevant commercial or (inter) national support programmes or funding.
Awareness of and enthusiasm for drug rediscovery should be boosted by encouraging international and national collaborative research, aimed to initiate studies on novel indications of existing drugs or apply new insights on pharmacodynamics, both by the scientific community as well as pharmaceutical entrepreneurs.