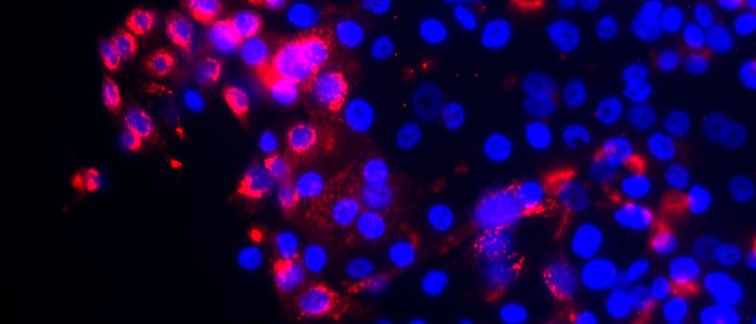
Cervical cancer is a heterogeneous disease arising from three cell populations. A cell-of-origin gene set could help identify misdiagnosed patients with tumours of a columnar origin, which are linked to worse prognosis. Uninfected reserve cells inherently exhibit a signalling phenotype which is induced by deregulation of human papillomavirus (HPV) oncogene expression in precancer and might thereby facilitate infection and/or transformation. This phenotype is also related to worse survival in cervical cancer.
Results described in the paper:
The expression of a cell-of-origin gene set in 279 cervical tumours derived from The Cancer Genome Atlas (TCGA) shows that cervical tumours can be divided into three groups based on their origin, with reserve cell tumours being the most common and having relatively good prognosis. The authors show, with experimental models of one-layer and multi-layered human skin, that high risk HPV drives a sustained upregulation of signalling protein DLL4 and a downregulation of DLL1. These changes appear to be associated with the increase in cell growth and division seen in cervical precancer. Reserve cells, which can be found in different areas of the cervix, are unique as immunostaining shows that they are characterised by this phenotype even before viral infection. In these uninfected cells these properties tend to disappear, as the increased cell growth and division is only temporarily needed. Importantly, this expression pattern could help identify other sites in the human body that might be equally susceptible to HPV-induced (pre)cancer formation. Importantly, tumours from 12 patients diagnosed as squamous-cell carcinoma, based on histology, showed a gene expression profile indicative of a columnar origin and were associated with poor prognosis. Additionally, high expression of DLL4 in cervical carcinomas was associated with worse survival. Both of these patient groups might benefit from more aggressive treatment.
Read the full paper on the website of Cancer Research