Neuropeptides are a group of signalling molecules that regulate many physiological functions such as brain development, synaptic plasticity, circadian rhythm, behaviours and emotions. Disruptions in neuropeptide systems are associated with pathological states, such as addiction, stress, major depression, schizophrenia, obesity and anorexia, antisocial behaviors and anxiety. Neuropeptides are secreted by neurons through the fusion of dense-core vesicles (DCVs), but the mechanisms DCV fusion has remained largely unknown.
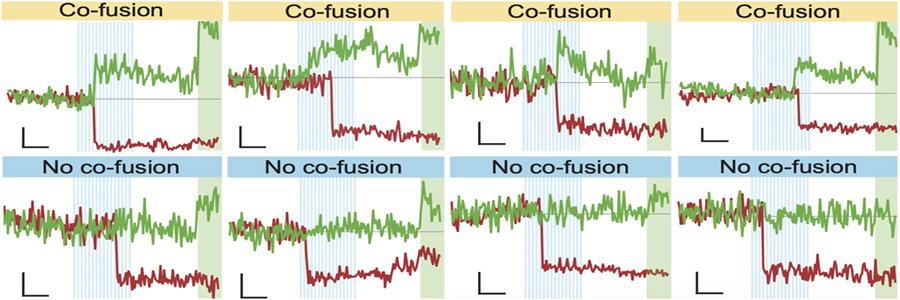
In this study, van Westen and her team identified two proteins, Synaptotagmin-1 (Syt1) and Synaptotagmin-7 (Syt7), that are required for DCV fusion. Synaptotagmins are proteins that bind Ca2+ and trigger membrane fusion. Inactivation of either Syt1 and Syt7 genes resulted in a strong reduction in DCV fusion. This could only be restored by expression of Syts that where capable to bind Ca2+. Deficiency of both Syts did not further reduce DCV fusion, suggesting that the two sensors operate in the same molecular pathway. Surprisingly, the two proteins could fully compensate for each other’s loss by expressing Syt1 in Syt7-deficient neurons, and vice versa, showing that Syt1 and Syt7 are functionally redundant. Overexpression in wild-type neurons increased DCV fusion, suggesting that both Syts are rate-limiting for DCV fusion. Together, these data show that the two calcium sensors regulate DCV fusion in an functionally redundant and expression level-dependent manner.
Read the paper in PNAS published on April 28, 2021 ‘Neuromodulator release in neurons requires two functionally redundant calcium sensors’.

