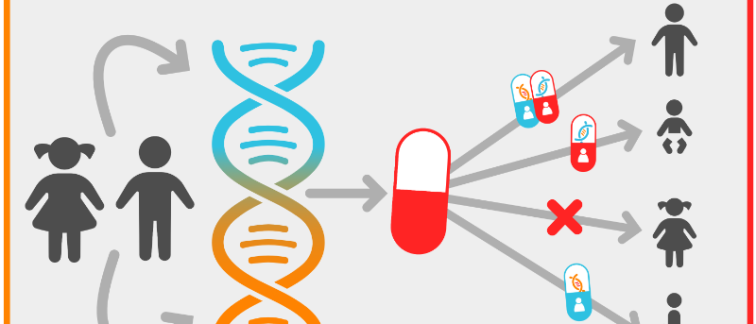
At the Emma Center for Personalized Medicine (Emma CPM), they accelerate the development, application, and evaluation of therapies for individuals with rare genetic diseases through a tailored approach. Established in 2022 at the Amsterdam UMC by co-directors prof. Clara van Karnebeek and prof. Mieke van Haelst, the Emma CPM aims to innovate and speed up diagnostics, personalized therapies and care for those with unmet medical needs. Their multidisciplinary team of (pre)clinical researchers and physicians facilitates the rapid translation of new knowledge and technology into personalized care.
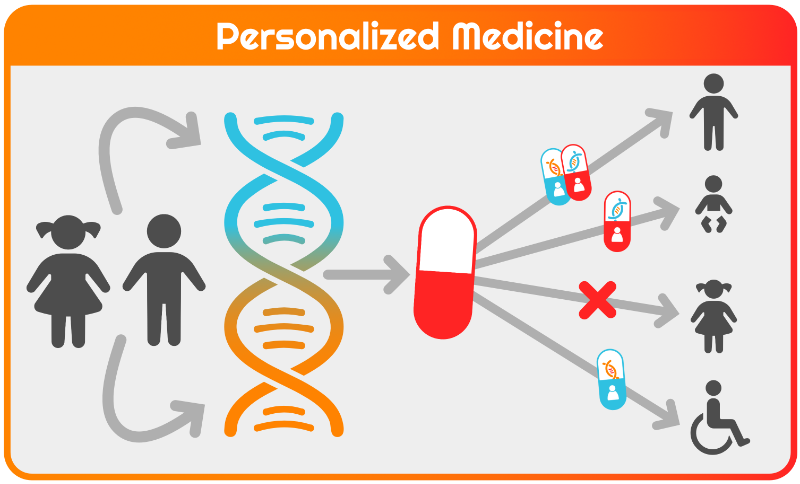
Emma CPM Therapy Clinic
The main goal of the Therapy Clinic is to bring promising therapies to the patient. Fast, safe, and accessible. The team, consisting of clinicians, trialists, methodologists, and a pharmacist, realizes efficient development, testing, and application of (novel) therapies focusing on nutritional therapies, (repurposed) drugs, and stem cell and RNA/gene therapies. The Therapy Clinic serves as a hub for conducting experimental trials, optimizing (targeted) therapy selection, and generating evidence of treatment effectiveness.
They apply a variety of therapies to different diseases in clinical care or research settings (clinical trials); such as mannose suppletion in a child with a congenital disorder of glycosylation, FCSK-CDG; L-Serine for children with loss-of-function of the GRIN2B gene; and cognate amino acid suppletion in children with aminoacyl-tRNA synthetases (ARS) deficiencies.
They also provide expert advice on the best possible therapy, how evidence can be generated considering innovative study designs, and how progress should be monitored, including relevant personalized outcome measures. In our monthly multidisciplinary Therapy Discovery Clubs, they discuss the best possible therapies case based. They support physicians by providing protocol templates, knowledge, skills, and resources for single-case (experimental) studies. By using master protocols, they enhance efficiency, reduce costs, accelerate trial development, and address ethical considerations.
The ‘indicating-performing-monitoring’ triad
Titel
Titel
They employ the ‘indicating-performing-monitoring’ triad to tailor the right therapeutic strategy for the right person at the right time, based on their unique phenotypes and genotypes. This triad addresses: 1) identifying the best possible therapy, based on evidence of target mechanism and therapy (cellular, animal model, clinical), pathophysiology, patient characteristics and the clinical need; 2) selecting an appropriate study design, considering the desired level of evidence, patient burden, pharmacovigilance, and suitable comparators; and 3) defining relevant endpoints, including type, relevance, validity, and responsiveness of outcome measures, frequency of measurements, interim analyses, and data collection.
Written by Annelieke Muller & Eva van Walree, two assistant professors supported by the Ministry of OCW sectorplan funds "Accelerating on Health” program to solve bottlenecks in the rare diseases therapy bench to bedside pipeline.

Annelieke Muller

Eva van Walree