Emphasis is on disease-oriented, translational research which is aimed at
- increasing mechanistic insight;
- developing specific diagnostic tools aimed at invasive and non-invasive assessment of microvascular health in different tissues;
- developing patient-tailored prevention together with improved treatment modalities targeted at the microcirculation.
Excellent research in search for effective treatment of vascular diseases
The endothelium is the largest, and at the same time, most diffuse organ in the human body. Endothelial cells control oxygenation, organ function and -perfusion and mediate the communication between blood and tissue.
Because endothelial cells line all vessels, they are exposed to systemic soluble and mechanical factors including cytokines and growth factors, blood composition as well as extracellular vesicles and hemodynamic force.
Conversely, the endothelium reflects the tissues’ condition and communicates with downstream sections of the circulation and tissues, through
- the release of extracellular vesicles;
- surface molecules
- the secretion of cytokines.
It is well established that the endothelium is heterogeneous and that tissue-specific differences between endothelial cells may explain altered sensitivity to circulating factors. Yet, many aspects of endothelial/microvascular pathologies (such as inflammation or increased permeability; perfusion and delivery; functional connection with the macrovasculature) appear similar between different organs.
Smooth muscle cells are important for the visco-elasticity of bloodvessels and play a role in vascular integrity. Loss of smooth muscle cell function could cause acute and chronic vascular diseases.
In search for effective treatment, future research should therefore focus at the tissue-specificity of endothelial (dys-) function as part of a strong line of translational research aimed at endothelial function in acute and chronic disease.
Infographics on research performed within the Microcirculation research program:
Main pathologies
- Ischemia-reperfusion injury
- Vascular inflammation
- Obesity-induced vascular dysfunction
- Vascular permeability and edema
- Endothelial dysfunction
- Obstructive (atherosclerotic) microvascular disease
Unique research expertise
- Live cell confocal- and superresolution microscopy and image analysis
- Analysis of angiogenesis/vascular sprouting in 3D
- 7 Tesla preclinical MRI and PET-imaging for mouse and rat
- Biobanking (e.g. Parelsnoer)
- Endothelial cell culture under hyper/hypoxic conditions
- Ex vivo analysis of (insulin-mediated) vascular function
- In vivo mouse and rat models for (microcirculatory) perfusion, (neuro) inflammation, stroke/cerebral hypoperfusion, vascular endothelial permeability
- Ultrasound-based analysis of tissue perfusion
- Biochemical analysis of protein activation, modifcation and degradation
- Intracoronary flow and pressure measurements to assess microvascular function
- Identification and prognostic implications of coronary vasospasms
Aim
The Research Program Microcirculation aims to aggregate excellent research on the vascular wall, focusing on endothelial and smooth muscle cell biology, physiology and mechanobiology, which drives vessel formation, integrity and perfusion. Balanced, bi-directional communication between clinicians and fundamental researchers to develop relevant research projects will be a key feature.
Emphasis will be on disease-oriented, translational research which is aimed at
- increasing mechanistic insight;
- developing specific diagnostic tools aimed at invasive and non-invasive assessment of microvascular health in different tissues;
- developing patient-tailored prevention together with improved treatment modalities targeted at the microcirculation.
Mircocirculation focuses on vascular pathology associated with diabetes, RA, renal failure, aging, chronic coronary syndromes, shock, aortic pathologies: aneurysms and dissections, diastolic heart failure and cardiomyopathies. Also, we aim for analysis of impaired interstitial transport and clearance, e.g. in the brain, which is relevant for stroke as well as Alzheimer’s disease. Finally, the interaction between endothelial cells and pericytes, smooth muscle cells and, where relevant, epithelial cells (e.g. lung, kidney) is subject of research.
This way, we aim to further bridge ongoing cardiac- and vascular research lines and to create strong, unique niches for translational research. The microcirculation is part of all organ systems and is thus of relevance for all research in ACS. Consequently, PI’s that are linked to the research program Microcirculation also participate in research linked to any of the other research programs in ACS, making Microcirculation the most integrated program in ACS.
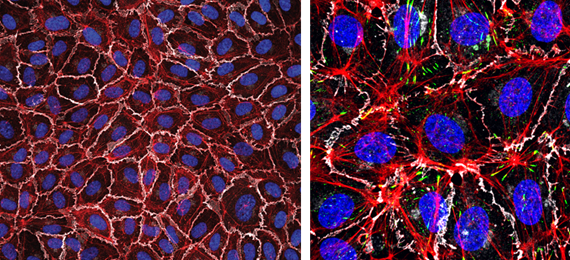 Left: Monolayer of human endothelial cells (White, VE-cadherin; red, F-actin; blue, nuclei). Right: zoomed image detailing F-actin in red, VE-cadherin in white and focal adhesions in green. Nuclei in blue.
Left: Monolayer of human endothelial cells (White, VE-cadherin; red, F-actin; blue, nuclei). Right: zoomed image detailing F-actin in red, VE-cadherin in white and focal adhesions in green. Nuclei in blue.
Program Leaders
Young ACS
Kak Khee Yeung & Jeffrey Kroon
Research themes
Oxygenation and perfusion
Throughout the body, the vasculature, and in particular the endothelium, is exposed to different levels of oxygen. A lack or excess of oxygen can have damaging effects on endothelial cells. Vascular occlusion, for example after a thrombotic event in the heart or brain, will lead to hypo-perfusion and ischemia, followed, upon treatment, by reperfusion. This reperfusion induces endothelial activation, production of reactive oxygen species, endothelial damage and increased vascular leak.
Obesity, insulin resistance and diabetes are closely related to cardiovascular diseases such as atherosclerosis, hypertension and heart failure. Microvascular functions such as control of blood flow and inflammation contribute to heart failure with preserved ejection fraction (HFpEF), but also to failure of other organs such as the brain, muscles and kidneys.
In addition to study microvascular perfusion in 3D, we study the role of fat tissue around blood vessels (perivascular adipose tissue, PVAT) in these cardiovascular diseases. Finally, we study visualization of perfusion in animal models by intravital microscopy, as well as in humans using innovative non-invasive imaging techniques.
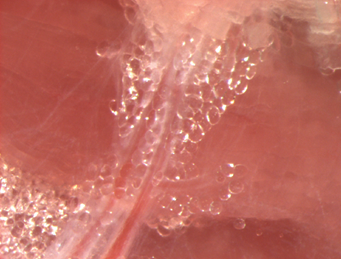 Human peri-vascular adipose tissue (PVAT)
Human peri-vascular adipose tissue (PVAT)
Inflammation and permeability
Endothelial dysfunction is commonly associated with, and caused by inflammation. We study various aspects of inflammatory responses, including the analysis of endothelial permeability, upregulation of inflammatory markers such as leukocyte adhesion receptors and expression of specific regulatory proteins.
These all affect cell-cell interactions between endothelial cells, and thus microvascular perfusion Vascular-Endothelial cadherin is the central adhesion molecule linking endothelial cells, and its regulation by phosphorylation as well as actin dynamics is a one of our main interests.
The consequent interactions of leukocytes and platelets with the activated endothelium is directly relevant for local inflammation, edema and tissue damage. This topic is studied in vitro using primary human endothelium and we also use zebrafish embryos and mouse models for analysis of vascular permeability and leukocyte-endothelium interactions.
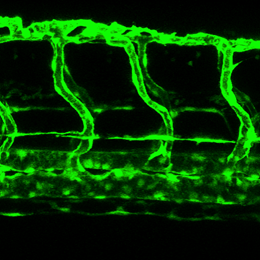 Zebrafish blood vessels
Zebrafish blood vessels
Mechano-biology and ageing
The effects of local forces, induced by flow, on the endothelium has direct and significant impact on vascular permeability, inflammation and perfusion. These forces are dependent on flow patterns, are altered by ischemia-reperfusion, but also modulated by systemic disease associated with hypertension.
Local changes in flow may result in increased vascular stiffness, which is pro-inflammatory. We study the role of biomechanics at the vascular, cellular and molecular level in relation to reactivity and permeability.
Altered vascular stiffness is also relevant for development of aneurysms and in we therefore also study vascular smooth muscle cell phenotype and contractility.
Another independent risk factor for cardiovascular disease is aging. Reduced microvascular endothelial function, potentially consequent to chonic, low-level systemic inflammation, is associated with aging and our research aims to unravel its underlying molecular mechanisms
Inflammatory response following ischaemia/reperfusion injury
Following acute myocardial infarction (AMI), an adequate healing response is crucial for preserving left ventricular (LV) function and geometry, and thus preventing adverse LV remodelling in patients.
Infarct healing is a complex and dynamic process, consisting of removing necrotic myocardium to replace this with scar tissue. During this process, a wide range of inflammatory cells dominate and monocytes in particular have recently drawn considerable attention as a target to improve healing following AMI.
The cardiology department at location AMC collaborates for this project with the pathology department at location VUmc to evaluate the infiltration of the wide range of inflammatory cells in myocardial tissue specimens of AMI patients.
Additionally, we focus on determining clinical characteristics of inflammatory cells in the peripheral blood of AMI patients to predict the development of adverse LV remodelling.
Microvascular involvement in ischemic heart disease
Since the introduction of the coronary angiogram, the identification and treatment of ischemic heart disease has been dominated by epicardial approaches.
Accumulating evidence suggest involvement of the coronary microcirculation in the manifestation of ischemic heart disease, which is frequently being overlooked in contemporary guidelines of myocardial ischemia. With the use of combined intra-coronary pressure (FFR) and flow measurements (CFR),
we aim to study the respective contribution of coronary and microvascular involvement in ischemic heart disease, the magnitude of microvascular resistance in the setting of NST-ACS In addition, we aim to investigate the independent prognostic value of combined FFR and CFR measurement in order to develop and implement tailored treatment strategies for patients with stable heart disease.
Coronary vasospasm (endothelial dysfunction)
A substantial number of patients with angina symptoms display no obstructive coronary artery disease on the diagnostic coronary angiogram. Vascular smooth muscle hyper-activity is considered one of the primary causes of endothelial-dependent dysfunction, which is associated with temporal epicardial and microvascular spastic vasomotion.
Being frequently overlooked in the diagnosis of ischemic heart disease, patients with endothelial-dysfunction show persistent misunderstood angina and associated with a poor quality of life and profound healthcare costs.
We study the application the acetylcholine provocation test to identify spontaneous coronary vasomotion and study the prognostic long-term implications of patients with a positive acetylcholine provocation test demonstrating coronary epicardial or microvascular endothelial dysfunction.
PI's and ongoing research lines
The following PI's are member of the Microcirculation Research Program:
| Principal Investigator | Location | Department |
|---|---|---|
| Peter Hordijk (program leader) | VUmc | Physiology |
| Erik Bakker (program leader)/Ed van Bavel | AMC | Biomedical Engineering and Physics |
| Jaap van Buul | Sanquin | Molecular Cell Biology lab |
| Charissa van den Brom | VUmc | Anesthesiology |
| Arjan Griffioen/Else Huijbers | VUmc | Medical Oncology |
| Victor van Hinsbergh/Pieter Koolwijk | VUmc | Physiology |
| Stephan Huveneers | AMC | Medical Biochemistry |
| Reinier Schlingemann/Ingeborg Klaassen | AMC | Ophthalmology |
| Elga de Vries | VUmc | Molecular Cell Biology and Immunology |
| Kakkhee Yeung | VUmc | Vascular Surgery |
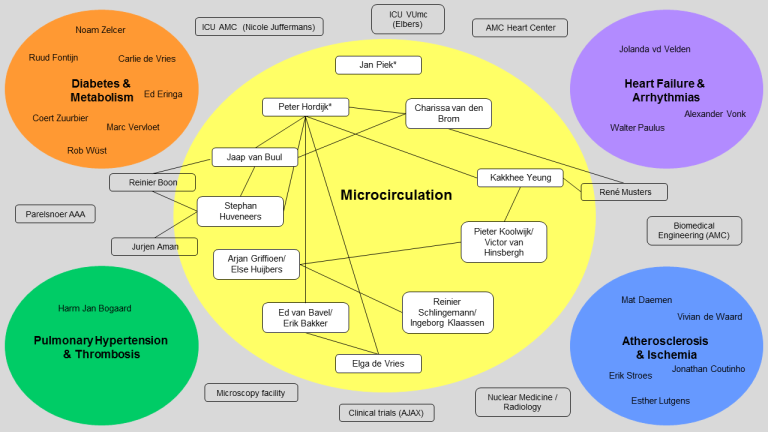
PI’s and staff members of the Microcirculation Research program were invited to give a short pitch about their research, funding and future plans for the coming years. This resulted in the figure presented below and an overview of missions along with slides for individual research lines (see Downloads). If your research is missing, please send an email to acs@amsterdamumc.nl with the ongoing research template (see Downloads).
Downloads
ACS research lines by Microcirculation Principal Investigators:
Program members
-
 Alexander VlaarPI Prof. MD PhD
Alexander VlaarPI Prof. MD PhD -
Amber Schonk
-
Anke van Bergen
-
Anne Beukers
-
Annett de HaanBEng
-
Bart CornelissenMSc
-
Bas BossersDR.
-
Bram van Steen
-
Can IncePI Prof. PhD
-
 Carolien VollemanBSc.
Carolien VollemanBSc. -
 Charissa van den BromPI DR.ING.
Charissa van den BromPI DR.ING. -
Charles MajoiePROF.DR.
-
Chi HauBA BEng
-
Christa BoerPI PROF.DR.
-
Cornelis AllaartPI PROF.DR.
-
Daan Wisselink
-
 Daphne NaessensMSc
Daphne NaessensMSc -
Ed van Bavel
-
Elmar GoolMEng MSc
-
 Erik BakkerPI PhD
Erik BakkerPI PhD -
Erik SernéPI DR.
-
 Eva AalbregtDRS.
Eva AalbregtDRS. -
 Fatimah Al Darwish
Fatimah Al Darwish -
Geert StreekstraPI MEng PhD
-
Gerard TangelderPROF.DR.
-
 Harm-Jan de GroothDR.
Harm-Jan de GroothDR. -
Haryadi PrasetyaMEng
-
 Helga de VriesPI PROF.DR.
Helga de VriesPI PROF.DR. -
Ijsbrand ZijlstraDR.
-
 Ilse Wissink
Ilse Wissink -
 Ingeborg KlaassenPI PhD
Ingeborg KlaassenPI PhD -
Ivo Jansen
-
 Iwan DobbePhD
Iwan DobbePhD -
Jaap van BuulPI
-
Jan BlankensteijnPROF.DR.
-
Jan S.M. van Bezu
-
 Jan VoorbergPI Prof. PhD
Jan VoorbergPI Prof. PhD -
Janina SchwarzMSc
-
 Jeffrey KroonPI PhD
Jeffrey KroonPI PhD -
Joanna van WijkDR. MD PhD
-
 Judith de VosBSc
Judith de VosBSc -
 Kak Khee YeungPI DR.
Kak Khee YeungPI DR. -
Karin Morsing
-
Katinka van Kranendonk
-
Laurens HuismanDR.
-
Liffert VogtPI Prof. MD PhD
-
Lothar SchwarteDR.
-
Lotte Rijken
-
 Ludo BeenenMD PhD
Ludo BeenenMD PhD -
Maarten Graumans
-
Manon KappelhofMD
-
 Marcus SchultzPI Professor Prof. MD PhD
Marcus SchultzPI Professor Prof. MD PhD -
Marjet Oppelaar
-
 Markus HollmannPI Prof. MD PhD
Markus HollmannPI Prof. MD PhD -
Martijn Klaver
-
Martijn van MourikMSc
-
Matthias Hilty
-
Max HoebinkDRS.
-
 Meagan DoppegieterMSc
Meagan DoppegieterMSc -
Nadia Hakkenbrak
-
 Natal van RielPI Prof. PhD
Natal van RielPI Prof. PhD -
Natalie LeCouffe
-
Nerea Arrarte Terreros
-
Noelle Bakker
-
 Paola Serrano Martinez
Paola Serrano Martinez -
 Patrick SchoberPI PROF.DR. PROF. DR.
Patrick SchoberPI PROF.DR. PROF. DR. -
Peter Hordijkprof. dr.
-
Philipp Hauger
-
Pieter KoolwijkDR.
-
Pieter Roel TuinmanPROF.
-
 Praneeta Konduri
Praneeta Konduri -
 Reinier BoonPI PROF.DR.
Reinier BoonPI PROF.DR. -
René van den BergPhD
-
Renee Helmers
-
Rianne Schoon
-
Richelle Hoveling
-
Rienk NieuwlandPI PhD
-
 Rik Olde EngberinkPhD
Rik Olde EngberinkPhD -
 Rio Juni
Rio Juni -
Robert WeeninkDoctor of Philosophy DR.
-
Rosa WoudaMSc
-
Ruggero Belluomo
-
S.E.C.M. van de Vijfeijken
-
Sahar Moukadem
-
Sani Kreca
-
Sanne ten Berg
-
Sha Chen
-
 Stephan HuveneersPI PhD
Stephan HuveneersPI PhD -
 Tariq DamMSC.
Tariq DamMSC. -
Thei Steenvoorden
-
Valeria Guglielmi
-
Valérie Stegehuis
-
Vera Janssen
-
Vincent JongkindDR.
-
Vincent Kurucz
-
Ward van der Ven
-
Willem WisselinkPI PROF.DR.
-
 Yen-Mie LaiDRS.
Yen-Mie LaiDRS. -
 Yolande AppelmanPI DR.
Yolande AppelmanPI DR. -
Zehra Uz



